In 2021, the adjustment of medical insurance catalogue is about to enter the declaration stage, and a number of domestic innovative drugs are expected to enter the medical insurance negotiations.
With the arrival of the deadline for the approval of drugs that can be examined on June 30, the adjustment of the national medical insurance drug list in 2021 is about to enter the declaration stage. According to the incomplete statistics of the Economic Information Daily reporter, about 40 innovative drugs or new indications were approved from August 18, 2020 to June 30, 2021, which means that a number of domestic innovative drugs are expected to enter the medical insurance negotiations. The market is highly concerned about the opportunities brought by the inclusion of innovative drugs in medical insurance. Institutions generally believe that the normalization of medical insurance catalogue adjustment will help the rapid increase of innovative drugs.
Innovative drugs were intensively approved in June.
According to the National Medical Insurance Bureau’s Work Plan for the Adjustment of the National Medical Insurance Drug List in 2021 (Draft for Comment) (hereinafter referred to as the "Plan"), after the preliminary preparation stage, it will enter the declaration stage from July to August, in which the enterprise’s declaration time is planned to be from July 1 to 14, and negotiations are expected to start from September to October, and the adjustment results will be announced from October to November. According to the "Program", new drugs or new indications approved from January 1, 2016 to June 30, 2021 have the opportunity to participate in medical insurance negotiations.
The Economic Information Daily reporter combed the institutional data and the announcements of listed companies and found that since the last round of medical insurance catalogue was included in the drug certification deadline (August 17, 2020), there were about 40 domestic innovative drugs that were approved for listing or added indications. In particular, the pace of listing innovative drugs has been further accelerated this year. In June alone, at least seven domestic Class I innovative drugs were approved for listing, involving many listed companies such as Hengrui Pharma, Aidi Pharmaceutical, Haizheng Pharmaceutical, Zejing Pharmaceutical and Rongchang Bio. The latest report released by National Medical Products Administration shows that in the whole year of 2020, the Drug Testing Center reviewed and approved 20 varieties of innovative drugs and new drugs for listing (NDA).
On June 28th, Aidi Pharmaceutical announced that the company had obtained the Drug Registration Certificate of Ainuoweilin Tablets approved and issued by National Medical Products Administration on June 25th, 2021. According to the disclosure, this product is Aidi Pharmaceutical’s first Class 1 new drug against AIDS, which is used to treat newly treated patients with HIV-1 infection.
On the same day, Haizheng Pharmaceutical disclosed that the company’s Haibo Maibu tablets had obtained the Drug Registration Certificate. As an auxiliary treatment other than diet control, this product can be used alone or in combination with HMG-CoA reductase inhibitors (statins) to treat primary (heterozygous familial or non-familial) hypercholesterolemia.
On June 23, Hanson Pharmaceutical disclosed that the first innovative drug, Amitenofovir Tablets, was approved for marketing. This product is an anti-infective drug and is suitable for the treatment of adult patients with chronic hepatitis B.
Earlier, Hengrui Pharma’s TPO receptor agonist Hatroppa ethanolamine tablets, Zejing Pharmaceutical’s anti-tumor drug Donafenib Toluene Sulfonate tablets, Rongchang Bio-targeted HER2 antibody conjugate (ADC) for injection, and Mengke Pharmaceutical’s antibacterial drug Contizontamide tablets were successively approved for listing.
According to industry analysts, innovative drugs or indications that meet the adjustment scope of medical insurance catalogue in 2021 include PD-1 monoclonal antibody, PARP inhibitor, ADC and so on.
It is worth noting that four domestic PD-1 products that entered the medical insurance catalogue in the previous two rounds of medical insurance negotiations all added new indications. On June 28th, Cinda Bio announced that Cindilizumab was approved as the fourth indication, and combined with bevacizumab injection (Dayoutong) was used as the first-line treatment for unresectable or metastatic hepatocellular carcinoma that had not received systematic treatment before. On June 23rd, Baekje Shenzhou announced that two new indications have been obtained for tirelizumab. On June 10th, Hengrui Pharma disclosed that Karelizumab was approved for the sixth indication. Previously, the application of Junshi Bio-Treprilizumab for the marketing of new indications for the treatment of patients with recurrent or metastatic nasopharyngeal carcinoma who had previously failed to receive second-line or above systemic treatment was conditionally approved.
Normalization adjustment is good for innovative drugs
At present, the inclusion of medical insurance catalogue has become a catalyst for innovative drugs to open up the incremental market. Take Cinda Bio as an example. In 2019, Dabaoshu of Cinda Bio became the only PD-1 monoclonal antibody product that entered the national Class B medical insurance at the price of 2,843 yuan (10 ml: 100 mg/bottle) during the medical insurance negotiation, which was 63.73% lower than the price of 7,838 yuan at the time of listing. In 2020, Daboshu, which was included in medical insurance, achieved a sales income of 2.29 billion yuan, accounting for nearly 60% of the company’s revenue that year.
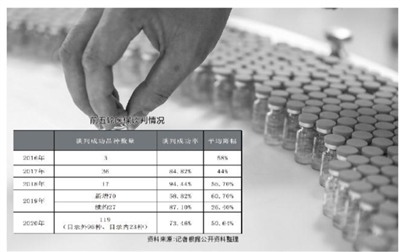
However, entering the medical insurance negotiations also means that the result of price reduction is already doomed. According to official data, in 2020, medical insurance negotiations involved 162 kinds of drugs, and finally 119 kinds of drugs were successfully negotiated, including 96 exclusive drugs, with a success rate of 73.46% and an average price reduction of 50.64%. In 2019, 119 new varieties were negotiated in medical insurance negotiations, and 31 varieties were continued in 2017, totaling 150 varieties. In the end, 97 varieties were successfully negotiated, of which the average price of 70 new varieties decreased by 60.7% and the average price of 27 successful varieties decreased by 26.4%.
The agency believes that in the past, because of its high pricing and long market access time, the market penetration rate of innovative drugs was low, but after being included in medical insurance, innovative drugs were quickly released. After the Medical Insurance Bureau began to implement the negotiation of medical insurance catalogue, the speed of bringing innovative drugs into medical insurance accelerated, which released a positive signal to the field of innovative drugs and benefited innovative pharmaceutical companies and industrial chains with strong research and development strength and rich pipelines.
Judging from the "Proposal", the adjustment of the medical insurance catalogue is still going in and out. Ping An Securities said that China’s medical insurance catalogue has established a dynamic adjustment mechanism, and the medical insurance fund can be used more effectively. China’s medical insurance catalogue adjustment time interval is constantly shortened, and the adjustment is more flexible. Considering the transfer mechanism of medical insurance catalogue, drugs with significant clinical value will be adjusted into the catalogue more quickly, while drugs with poor auxiliary drugs and drug economy will be transferred out, speeding up the change of medical insurance fund. The dynamic adjustment mechanism of medical insurance catalogue can accelerate the quantity of innovative drugs, help them seize the market better and improve the accessibility of new drugs.